The Hirst Lab has been awarded a new three-year NSF grant along with collaborator UC Merced’s Kevin Mitchell.
The grant, combining experiment and theory is titled “Self-mixing Active Fluids”
Abstract
Active matter is one of the most exciting frontiers in soft matter science. Unlike typical fluids, active fluids are not in equilibrium. Instead, they consume energy locally, translating this energy into internal flows and spontaneous mixing. In this project, mass transport and chaotic mixing in active fluids will be investigated using a fluid consisting of microtubules and kinesin, biological molecules found in the cell. Densely packed microtubules slide antiparallel to each other at a controlled rate due to coupled kinesin molecular motors. An exciting outcome of this work could be the development of a new class of self-mixing active solvents. Such a solvent could revolutionize our understanding of the kinetics of mass transport and chemical reactions. The present proposal concerns much larger length scales than that of standard solvents and will thus serve as an experimental model for these new ideas, helping to establish fundamental laws that govern the behavior of active matter. Since the contents of biological cells are highly complex active materials, far from equilibrium, this work is expected to yield new insights into the role of active materials in biology. A proposed Telluride workshop on transport in active fluids will help to bring together the relatively disparate fields of liquid crystals, biological fluids and nonlinear dynamics. This work will have a significant educational impact at UC Merced, a new university in one of California’s most socio-economically disadvantaged areas. This research will provide the basis for several undergraduate theses and the PIs will use insights from this work to introduce cutting edge materials to their graduate and undergraduate teaching.
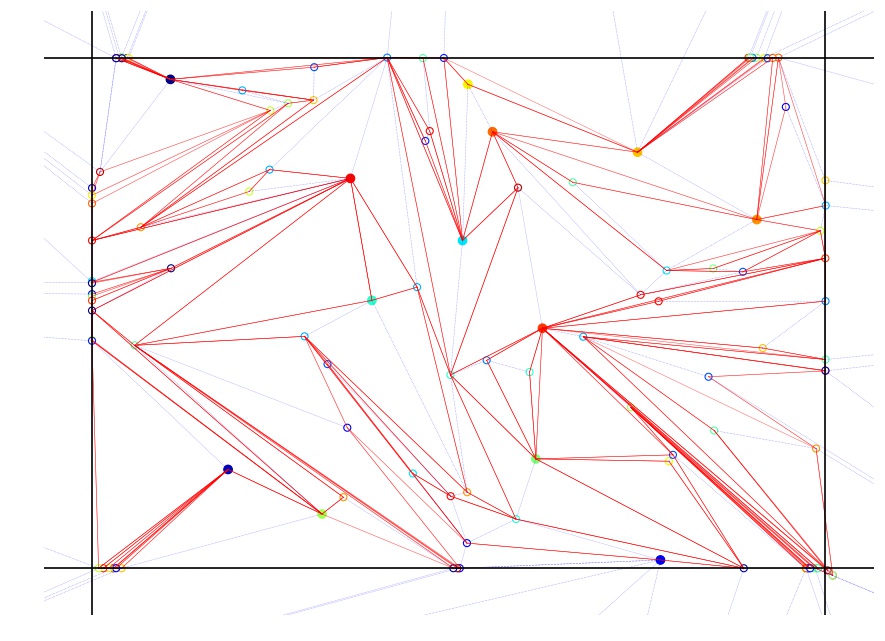
This project focuses on transport and mixing in a biologically inspired extensile active nematic. Densely packed microtubules slide antiparallel to each other at a controlled rate due to kinesin molecular motors and the resulting chaotic advection will be measured on different length scales, using the experimental tools of particle tracking, particle image velocimetry, and fluorescence imaging of labeled tracers. Experimental data will be theoretically interpreted using the tools of nonlinear and topological dynamics, thereby merging the fields of chaotic advection and liquid crystals in a unique collaborative effort. Topological entropy will play a central, unifying role in this study. Topological entropy is well known in studies of chaotic advection, but has been thus far overlooked in studies of active nematics. Specific aims are to: 1) use bead tracking and velocity reconstruction, together with tools from nonlinear dynamics, to measure the topological entropy of active nematic mixing; 2) measure the effective diffusivity, enhanced by chaotic advection, of the active nematic on the macroscale; and 3) investigate correlations between molecular-scale dynamics, mesoscale mixing, and macroscale diffusion by varying system parameters.





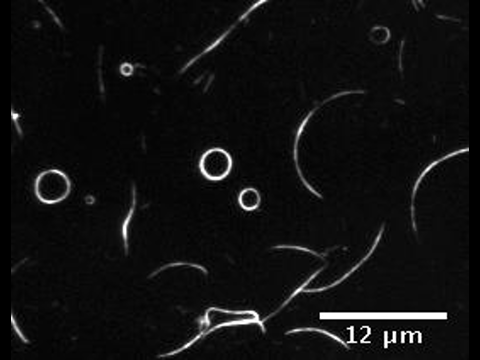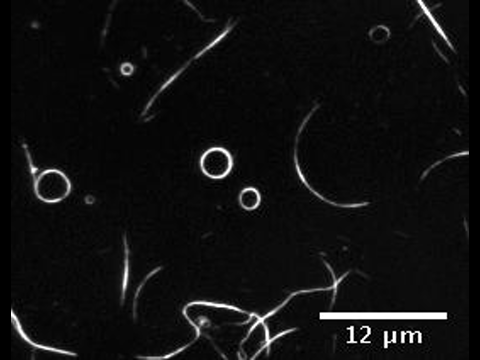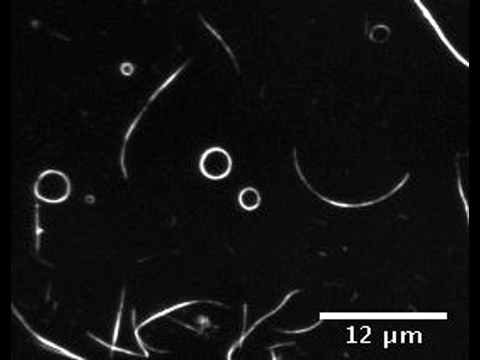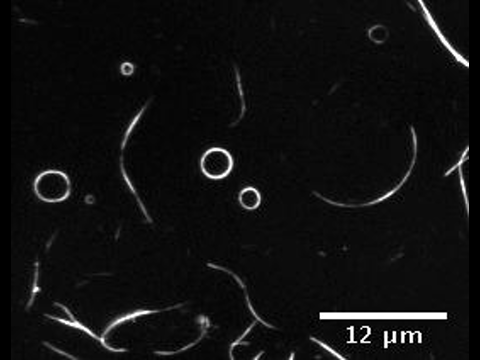
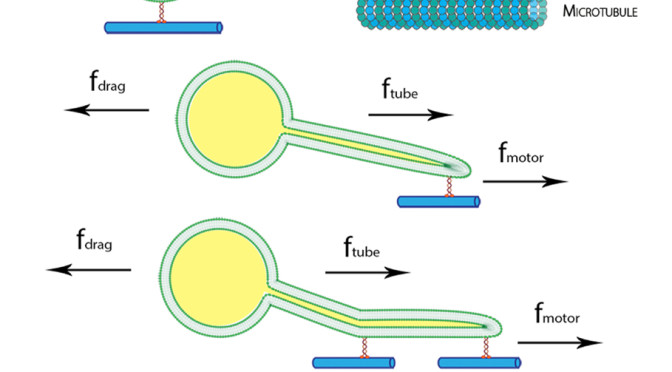
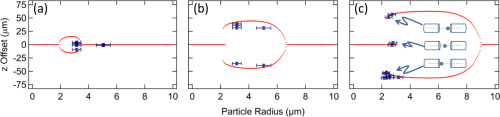
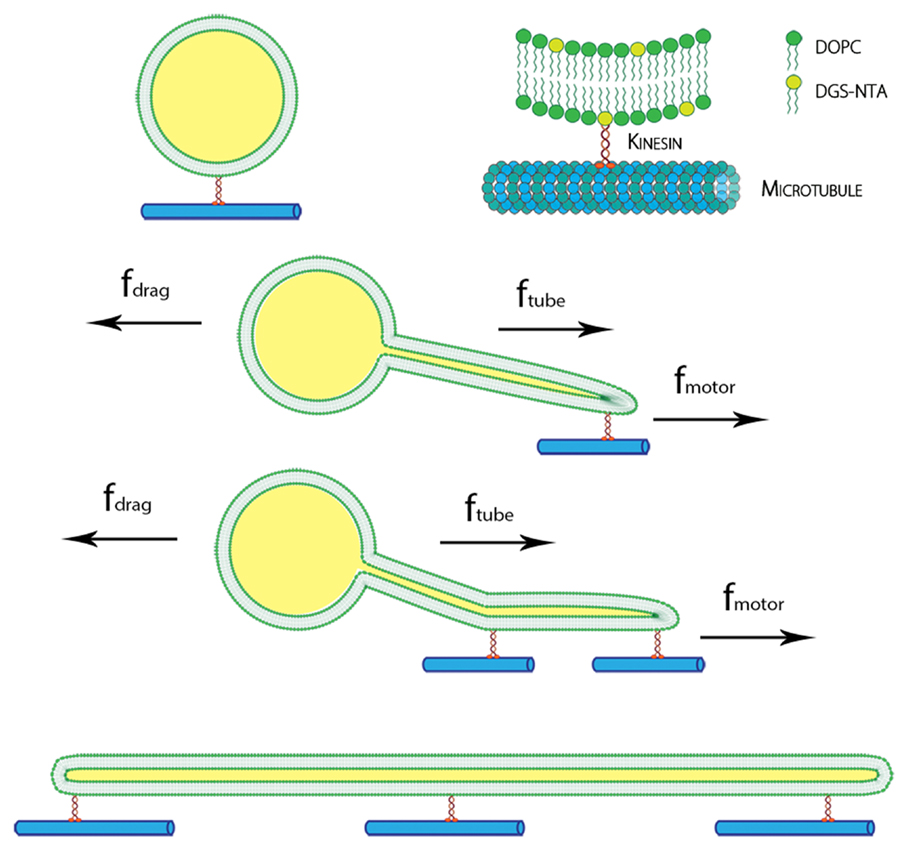 The presence of membrane tubules in living cells is essential to many biological processes. In cells, one mechanism to form nanosized lipid tubules is via molecular motor induced bilayer extraction. In this paper, we describe a simple experimental model to investigate the forces required for lipid tube formation using kinesin motors anchored to 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) vesicles. Previous related studies have used molecular motors actively pulling on the membrane to extract a nanotube. Here, we invert the system geometry; molecular motors are used as static anchors linking DOPC vesicles to a two-dimensional microtubule network and an external flow is introduced to generate nanotubes facilitated by the drag force. We found that a drag force of ≈7 pN was sufficient for tubule extraction for vesicles ranging from 1 to 2 μm in radius. By our method, we found that the force generated by a single molecular motor was sufficient for membrane tubule extraction from a spherical lipid vesicle.
The presence of membrane tubules in living cells is essential to many biological processes. In cells, one mechanism to form nanosized lipid tubules is via molecular motor induced bilayer extraction. In this paper, we describe a simple experimental model to investigate the forces required for lipid tube formation using kinesin motors anchored to 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) vesicles. Previous related studies have used molecular motors actively pulling on the membrane to extract a nanotube. Here, we invert the system geometry; molecular motors are used as static anchors linking DOPC vesicles to a two-dimensional microtubule network and an external flow is introduced to generate nanotubes facilitated by the drag force. We found that a drag force of ≈7 pN was sufficient for tubule extraction for vesicles ranging from 1 to 2 μm in radius. By our method, we found that the force generated by a single molecular motor was sufficient for membrane tubule extraction from a spherical lipid vesicle.